Vivianite Raman Spectrum.
|
|
Transparent section of a Vivianite mono crystal from Bolivia. This section has been used to record the absorption spectrum and the Raman spectrum below. The color of this mineral is rather pale. |
 |
| Other Vivianite specimen from Crimea. The color of
this Vivianite is intense blue due to high oxidation of the Iron.
|
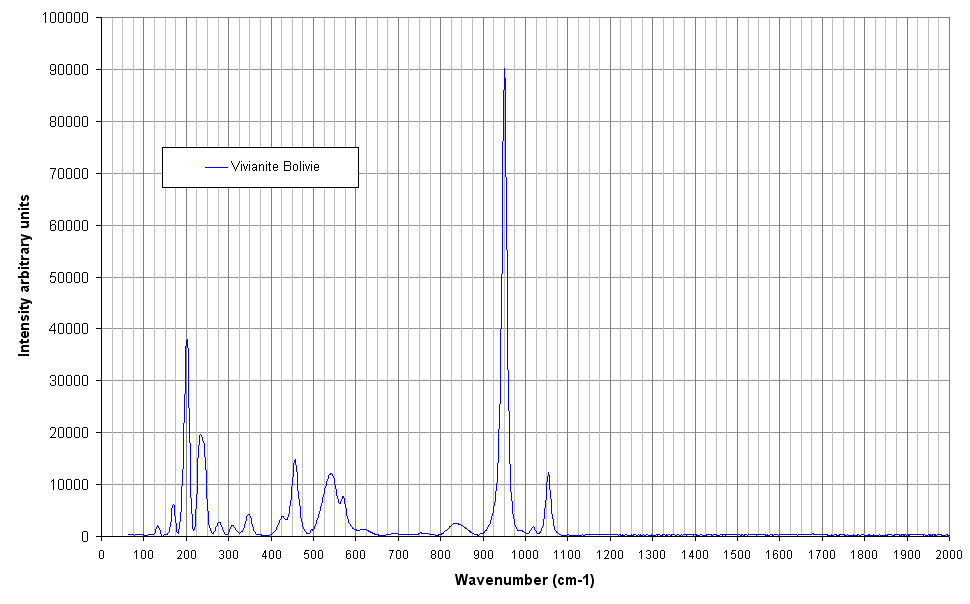 |
| Raman spectrum of Vivianite taken with the 633 nm
Laser. The highest peak at 950 cm-1 is the stretching
vibration of the phosphate group.
|
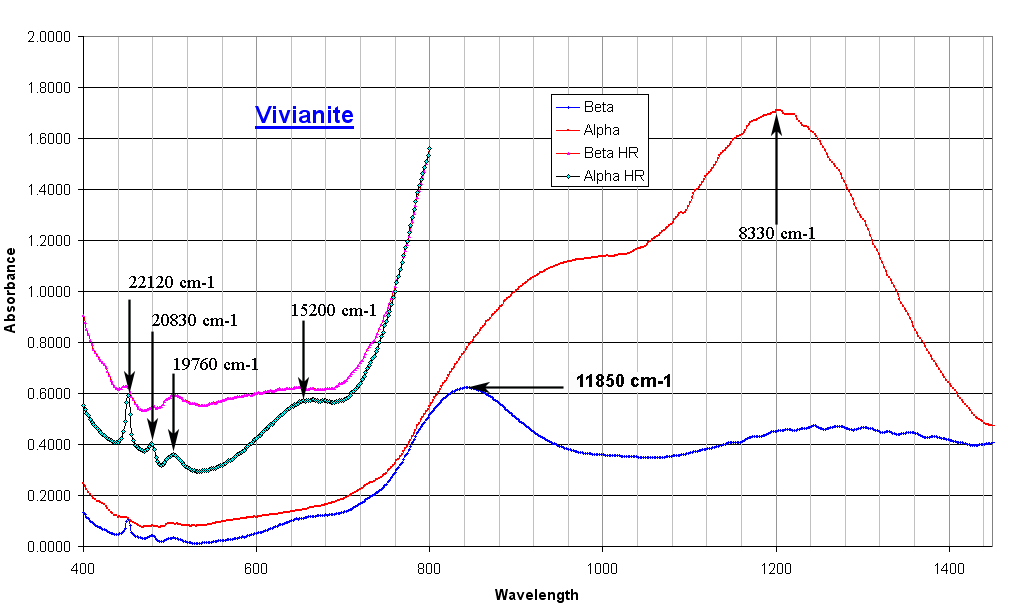 |
| Absorption spectra of a Vivianite single crystal pale
blue (top picture). The color of the Vivianite can be white to dark blue
according to the degree of oxydation of the Iron. When iron is Fe2+
the crystal is white. If some Fe2+ is oxidized to Fe3+,
the color is blue, the higher the oxidation the higher the intensity. The 2 bands at 8330 and 11850 cm-1 are crystal field transitions of the Fe2+ ion. The band at 15200 cm-1 is the intervalence charge transfer band between Fe2+ and Fe3+. The crystal examined here being only slightly colored, the intensity of this band is low. It appears along the beta orientation. If the degree of oxidation increases, the intensity of this band will rise and can dominate the spectrum. The small band at higher energy (19760 to 22120 cm-1) are spin forbidden Fe2+ crystal field transitions. |
