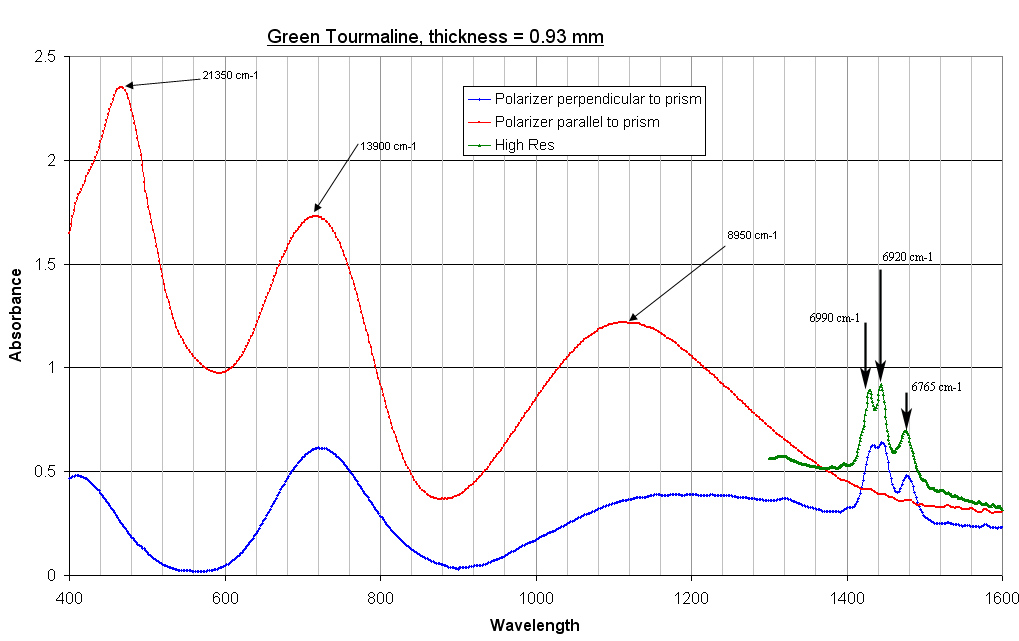
Tourmaline spectra.
|
|
Highly pleochroic section of green
tourmaline. Spectrum is mainly made up of 3 peaks: 700 and 1100 nm being
due to crystal field transitions within Fe2+ ions and 420-460
nm to charge transfer band. Fe2+ in tourmaline structure can
replace either Al3+ or Mg2+. The symmetry of these
sites being different, they give rise to transitions at different
frequencies. The triplet peak around 1420 nm is produced by the vibration overtones in hydroxyl radicals. Notice the absence of an H2O spectrum for a polarizer direction perpendicular to optic axis. The 3 H2O bands are the first harmonic of the stretching OH vibrations. The fundamental bands can be seen in the Raman spectrum of the same crystal. |
|
|
|
|
|
|